ABOUT
Lingrui Biotech provides automatic injectors, including both single-use and reusable injection pens, primarily focusing on drug delivery devices for diabetes and obesity patients. The company specializes in developing multi-dose injector pens and disposable auto-injectorsubcutaneous systems, ensuring high precision and reliability in the administration of GLP-1 and insulin medications.
Additionally, Lingrui Biotech offers semi-automatic assembly equipment for auto-injector pens, reusable insulin pens, and drug-delivery systems testing equipment, allowing for efficient production and assembly of injection pens. The company also provides solutions for the semi-automatic assembly of injectors and drugs, catering to the needs of pharmaceutical manufacturers and ensuring seamless integration between drug and injection devices.
Lingrui’s capabilities extend to ODNmulti-dose injector OEM services, delivering customized solutions for different pharmaceutical applications. Moreover, Lingrui focuses on the design and manufacturing of GLP-1 pen injectors, including both disposable and reusable options, ensuring a versatile approach to drug administration for both chronic GLP-1 injections and GLP-1 subcutaneous pen injectors.
With expertise in auto-injector assembly equipment and auto-injector Drug auto-injector OEM, Lingrui continues to provide state-of-the-art devices that improve patient experience and enhance medication delivery effectiveness.

PRODUCTS
-

3mL Cartridge Reusable Pen Injector
Read more -

Hidden Needle Reusable Pen Injector
Read more -

Reusable Pen Injector Support CE MDR & FDA (510k)
Read more -

Hot Selling Disposable 60U& 75U& 80U High Quality Pen
Read more -

3 mL Cartridge Multi-dose Auto Injector Pen
Read more -

3 mL Cartridge Multi-dose Auto Injector Pen
Read more -

3 mL Cartridge Disposable Injector Pen
Read more -

PFS-based Autoinjector Pen
Read more -
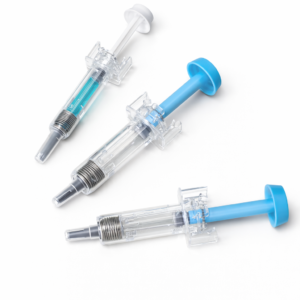
PFS Neddle Safety Device
Read more -

Smart Electronic Pen Injector
Read more -

auto injector automatic assembly line
Read more -

Auto injector testing device
Read more
ADVATAGES

Strong technological breakthrough
Domestic company with independent intellectual property rights, breaking foreign patent blockade, preferred partner for pharmaceutical companies.

Unique full industry chain
Large-scale industrial production capacity, self-developed auto-assembly and testing equipment for injection pens, expertise in drug-device combination applications.

Top industry team
Core team with cross-disciplinary backgrounds in mechanical design, automation, biological agents, materials science and artificial intelligence, deep understanding of self-medication device industry.
